Z01
A translational and computational platform for longitudinal studies of SCLC under therapy
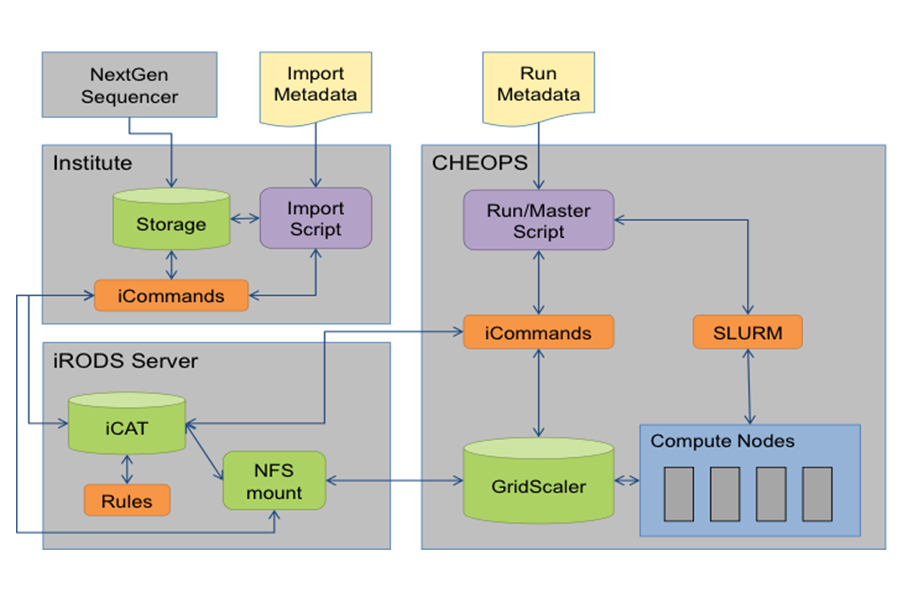
Z01 will provide platforms and technology to funnel clinical specimens that were collected longitudinally during the course of routine treatment of SCLC patients and as part of clinical trials. Z01 will therefore conduct a registry trial to collect clinical specimens, as well as all associated clinical data in a highly standardized fashion. Similarly, it will be critical to provide a computational platform to make this highly granular data available to all subprojects that seek to validate their findings in humans. Thus, Z01 will also provide database and –access technology that ensures searching the clinical and experimental data (e.g., from sequencing studies) in real time. Finally, Z01 will provide essential data analysis pipelines (e.g., for transcriptome sequencing) to the various subprojects that apply sequencing technology to mine their experimental samples.
Principal Investigator

Univ. Prof. Dr. med. Jürgen Wolf
Medical Director Center for Integrated Oncology CIO Köln
University Hospital of Cologne
Department I for Internal Medicine, Center for Integrated Oncology Köln Bonn (CIO)

Dr. med. Katja Höpker
Research Group leader
University Hospital Cologne
Clinic III for Internal Medicine